Cyclic Polymer Grafts that Protect Cartilage and Restore its Natural Function
A team of researchers led by Dr. Edmondo M. Benetti (Polymer Surfaces Group, D-MATL) and Prof. Marcy Zenobi-Wong (Tissue Engineering and Biofabrication, D-HEST) designed tissue-reactive graft copolymers to protect the cartilage against enzymatic degradation and restore its lubrication properties during the early stages of osteoarthritis (OA).
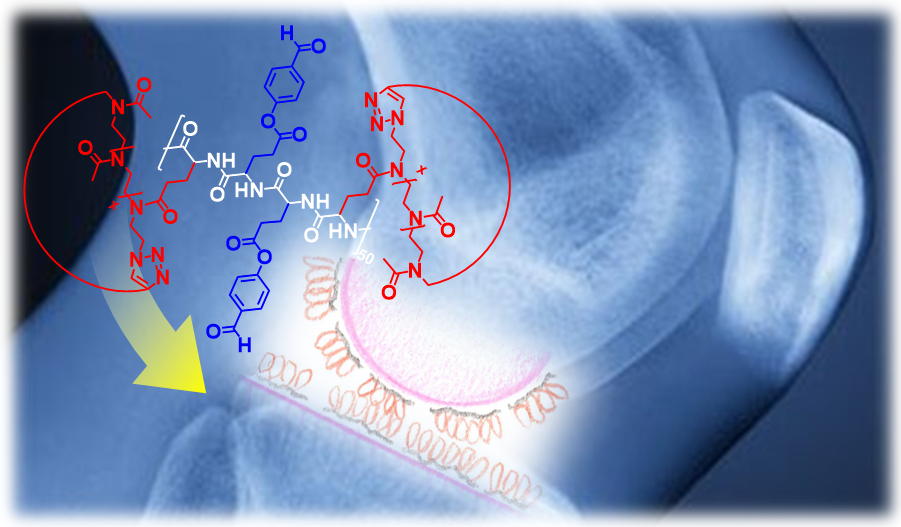
These copolymers feature a poly(glutamic acid) (PGA) backbone bearing hydroxybenzaldehyde (HBA) functions and cyclic poly(2-methyl-2-oxazoline) (PMOXA) side chains. PGA-PMOXA-HBA species chemisorb on the degraded tissue via Schiff bases and expose the biopassive and lubricious PMOXA cyclic grafts at the interface. The smaller hydrodynamic radius by cyclic PMOXA side chains coupled to the intrinsic absence of chain ends generate denser and more lubricious films on cartilage when compared to those produced by copolymers bearing linear PMOXA. These "polymer topology effects" demonstrate how the introduction of cyclic polymers within tissue-reactive copolymers substantially improve their tribological and biopassive properties, suggesting a plethora of possible applications for cyclic macromolecules in biomaterials formulations. The research coordinated by Benetti and Zenobi-Wong has been recently published by external page Angewandte Chemie.
- Dr. Edmondo M. Benetti, Polymer Surfaces Group
- Prof. Marcy Zenobi-Wong, Tissue Engineering and Biofabrication
- Morgese, G., Cavalli, E., Rosenboom, J.-G., Zenobi-Wong, M. and Benetti, E. M. (2018), Cyclic Polymer Grafts That Lubricate and Protect Damaged Cartilage. Angew. Chem. Int. Ed., external page doi:10.1002/anie.201712534