Au–Si Eutectic Alloy With Four Melting Temperatures
In a recent Advanced Science publication, Güven Kurtuldu and Jörg F. Löffler of the Laboratory of Metal Physics and Technology (LMPT) illustrate that metastable crystals can be isolated via ultrafast calorimetry. Investigating their thermophysical properties, they find that even a simple binary Au–Si eutectic alloy reveals four distinctly different melting points.
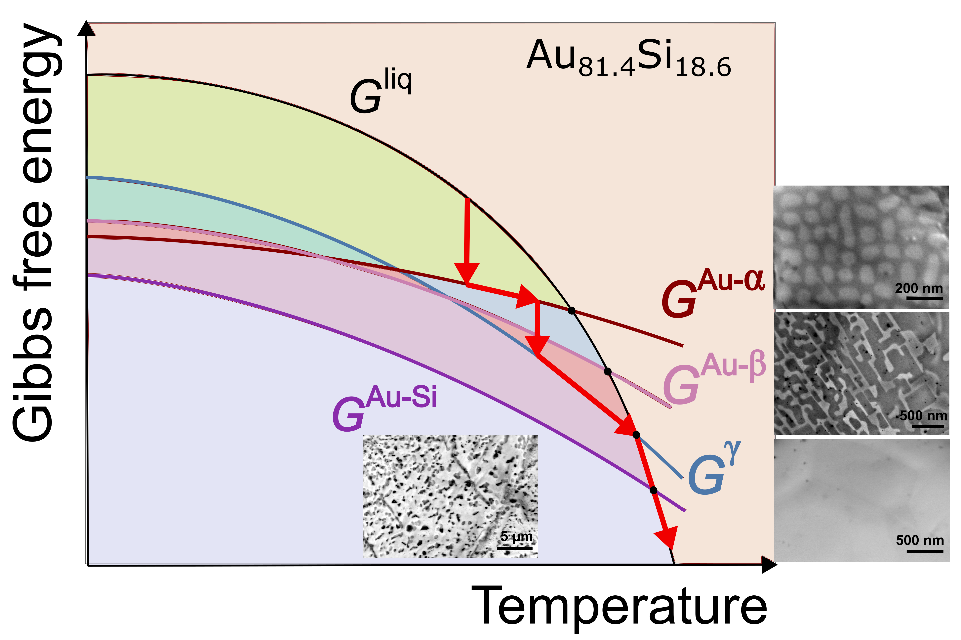
Crystals do eventually melt if they are heated to their characteristic melting temperature. However, because of the rapid nucleation and growth kinetics in metals, low-temperature metastable crystals generally transform upon heating into a more stable solid before they are able to melt. With the help of fast differential scanning calorimetry (FDSC) the authors have, however, shown in the Adv. Science article that it is possible to suppress this metastable-to-stable phase transition and thus to fully melt the metastable crystal. In this way they can determine the crystal’s detailed thermophysical properties such as melting temperature and enthalpy.
When they applied this strategy to a simple binary eutectic Au–Si alloy, the authors identified various metastable and stable solid states that all form under well-controlled conditions and that melt at four distinctly different melting temperatures ranging between 294°C and 363°C, with different melting enthalpies. Based on these data they were able to construct an energy–temperature diagram that describes the alloy’s multistep crystallization and melting pathways (see Figure).
This approach is a significant step in metals research because it advances the general understanding of nucleation in metallic and other systems. The construction of energy–temperature diagrams also makes possible detailed investigations into metastable phase formation and the synthesis of specific metastable materials. It thus sets a cornerstone for the discovery and design of metastable materials potentially useful in structural and functional applications.
- Multistep crystallization and melting pathways in the free-energy landscape of a Au–Si eutectic alloy, G. Kurtuldu and J. F. Löffler, Adv. Science 1903544 (2020) 1-7. external page DOI:10.1002/advs.201903544
- Laboratory of Metal Physics and Technology, ETH Zurich